Ice Table Vs Henderson Hasselbalch . Web the calculation of the ph of a buffer is straightforward using an ice table approach. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. If 0.3 < initial moles of base, the equivalence point has not yet been.
from www.numerade.com
Web the calculation of the ph of a buffer is straightforward using an ice table approach. If 0.3 < initial moles of base, the equivalence point has not yet been. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far.
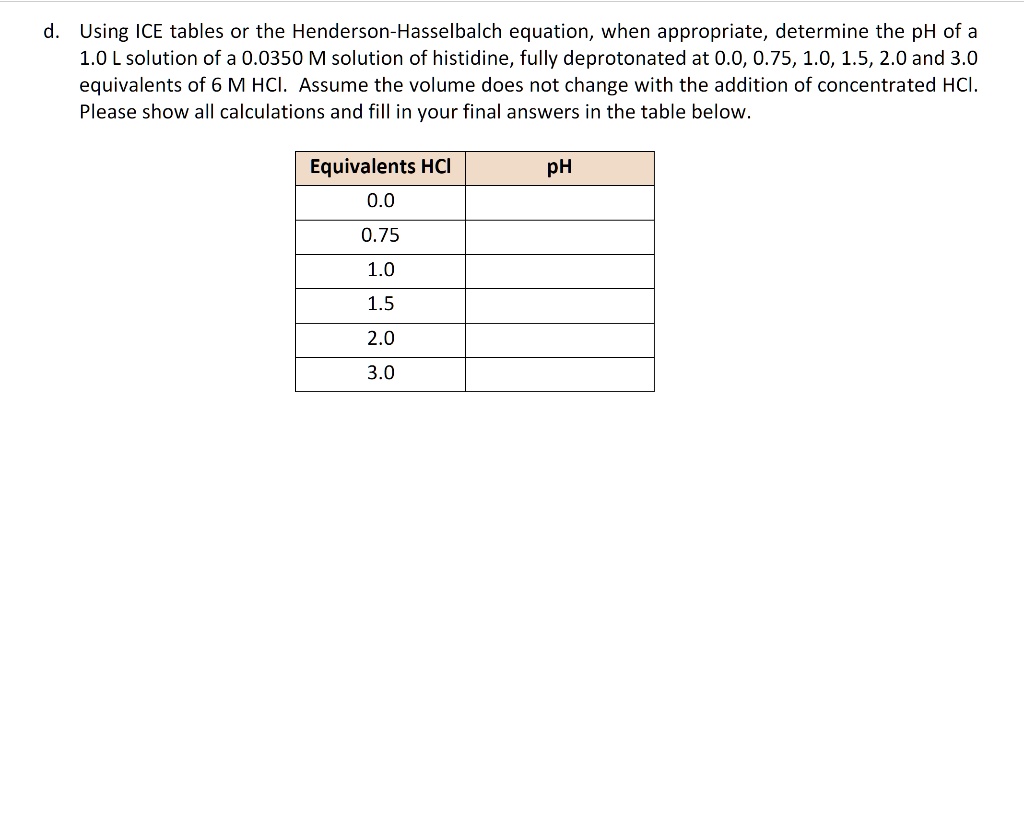
SOLVED Using ICE tables or the HendersonHasselbalch equation, when
Ice Table Vs Henderson Hasselbalch Web the calculation of the ph of a buffer is straightforward using an ice table approach. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Web the calculation of the ph of a buffer is straightforward using an ice table approach. If 0.3 < initial moles of base, the equivalence point has not yet been.
From www.numerade.com
SOLVED The answer is suppose to be 9.26Please show ICE table, steps Ice Table Vs Henderson Hasselbalch Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Web the calculation of the ph of a buffer is straightforward using an ice table approach. If 0.3 < initial moles of base, the equivalence point has not yet been. Ice Table Vs Henderson Hasselbalch.
From leah4sci.com
HendersonHasselbalch MCAT Trick for Buffer pH Without a Calculator Ice Table Vs Henderson Hasselbalch If 0.3 < initial moles of base, the equivalence point has not yet been. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Web the calculation of the ph of a buffer is straightforward using an ice table approach. Ice Table Vs Henderson Hasselbalch.
From schoolmoli.weebly.com
Ice chemistry calculator schoolmoli Ice Table Vs Henderson Hasselbalch If 0.3 < initial moles of base, the equivalence point has not yet been. Web the calculation of the ph of a buffer is straightforward using an ice table approach. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Ice Table Vs Henderson Hasselbalch.
From www.chegg.com
Solved 2. Use an ICE table and the Henderson Hasselbalch Ice Table Vs Henderson Hasselbalch Web the calculation of the ph of a buffer is straightforward using an ice table approach. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. If 0.3 < initial moles of base, the equivalence point has not yet been. Ice Table Vs Henderson Hasselbalch.
From www.slideserve.com
PPT pKa concepts PowerPoint Presentation, free download ID6594110 Ice Table Vs Henderson Hasselbalch Web the calculation of the ph of a buffer is straightforward using an ice table approach. If 0.3 < initial moles of base, the equivalence point has not yet been. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Ice Table Vs Henderson Hasselbalch.
From slideplayer.com
Acids Bases Equilibria Part V Buffers ppt download Ice Table Vs Henderson Hasselbalch If 0.3 < initial moles of base, the equivalence point has not yet been. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Web the calculation of the ph of a buffer is straightforward using an ice table approach. Ice Table Vs Henderson Hasselbalch.
From www.youtube.com
pH and pKa relationship HendersonHasselbalch equation YouTube Ice Table Vs Henderson Hasselbalch Web the calculation of the ph of a buffer is straightforward using an ice table approach. If 0.3 < initial moles of base, the equivalence point has not yet been. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Ice Table Vs Henderson Hasselbalch.
From www.numerade.com
SOLVED Using ICE tables or the HendersonHasselbalch equation, when Ice Table Vs Henderson Hasselbalch Web the calculation of the ph of a buffer is straightforward using an ice table approach. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. If 0.3 < initial moles of base, the equivalence point has not yet been. Ice Table Vs Henderson Hasselbalch.
From slideplayer.com
AP Chemistry Aqueous Equilibria, Part Two. ppt download Ice Table Vs Henderson Hasselbalch Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Web the calculation of the ph of a buffer is straightforward using an ice table approach. If 0.3 < initial moles of base, the equivalence point has not yet been. Ice Table Vs Henderson Hasselbalch.
From elchoroukhost.net
Ice Table Calculator Online Elcho Table Ice Table Vs Henderson Hasselbalch Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Web the calculation of the ph of a buffer is straightforward using an ice table approach. If 0.3 < initial moles of base, the equivalence point has not yet been. Ice Table Vs Henderson Hasselbalch.
From www.youtube.com
Example Calculate pH for a Weak Acid using an ICE Table YouTube Ice Table Vs Henderson Hasselbalch Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. If 0.3 < initial moles of base, the equivalence point has not yet been. Web the calculation of the ph of a buffer is straightforward using an ice table approach. Ice Table Vs Henderson Hasselbalch.
From www.pinterest.com
Buffer Chemistry Notes in 2021 Chemistry notes, Chemistry, Notes Ice Table Vs Henderson Hasselbalch If 0.3 < initial moles of base, the equivalence point has not yet been. Web the calculation of the ph of a buffer is straightforward using an ice table approach. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Ice Table Vs Henderson Hasselbalch.
From slideplayer.com
Buffers and HendersonHasselbalch ppt download Ice Table Vs Henderson Hasselbalch Web the calculation of the ph of a buffer is straightforward using an ice table approach. If 0.3 < initial moles of base, the equivalence point has not yet been. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Ice Table Vs Henderson Hasselbalch.
From www.numerade.com
SOLVED Understanding the source of the HendersonHasselbalch equation Ice Table Vs Henderson Hasselbalch Web the calculation of the ph of a buffer is straightforward using an ice table approach. If 0.3 < initial moles of base, the equivalence point has not yet been. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Ice Table Vs Henderson Hasselbalch.
From www.chegg.com
Solved 2. Use an ICE table and the Henderson Hasselbalch Ice Table Vs Henderson Hasselbalch If 0.3 < initial moles of base, the equivalence point has not yet been. Web the calculation of the ph of a buffer is straightforward using an ice table approach. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Ice Table Vs Henderson Hasselbalch.
From www.numerade.com
SOLVED Using ICE tables or the HendersonHasselbalch equation, when Ice Table Vs Henderson Hasselbalch Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. If 0.3 < initial moles of base, the equivalence point has not yet been. Web the calculation of the ph of a buffer is straightforward using an ice table approach. Ice Table Vs Henderson Hasselbalch.
From slideplayer.com
Applications of Aqueous Equilibria ppt download Ice Table Vs Henderson Hasselbalch If 0.3 < initial moles of base, the equivalence point has not yet been. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Web the calculation of the ph of a buffer is straightforward using an ice table approach. Ice Table Vs Henderson Hasselbalch.
From www.chegg.com
Solved ICE tables are used for calculating changes in Ice Table Vs Henderson Hasselbalch If 0.3 < initial moles of base, the equivalence point has not yet been. Web 0.050 l × 6 mol/l = 0.3 moles of strong acid added thus far. Web the calculation of the ph of a buffer is straightforward using an ice table approach. Ice Table Vs Henderson Hasselbalch.